
SpineNet is a complete solutions provider of spine fusion devices.



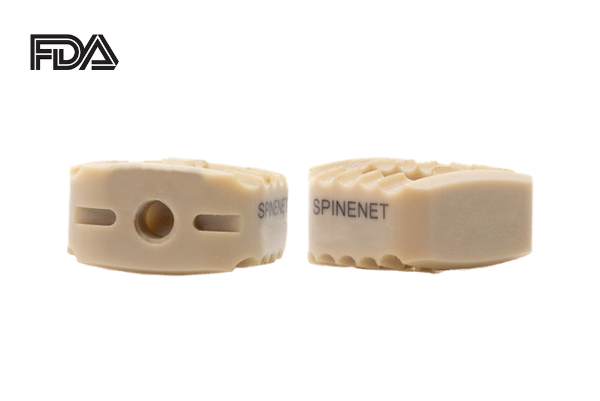
SpineNet, LLC announces the 510(k) U.S. FDA clearance of the SpineNet ACC System. The SpineNet ACC System is a system of wedge-shaped implants and instruments designed for anterior cervical interbody fusion. To prevent migration, the SpineNet ACC, has teeth on its superior and inferior surfaces.
The SpineNet ACC system is indicated for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one disc level. The SpineNet ACC implants are used to facilitate intervertebral body fusion in the cervical spine and are placed via an anterior approach at the C2 to T1 disc levels using autograft bone. The SpineNet ACC implants are to be used with supplemental fixation.